Single-Molecule Localization Microscopy (SMLM)
Super-resolution imaging beyond the diffraction limit
Single-Molecule Localization Microscopy (SMLM) is a powerful optical technique that enables imaging of biological structures with nanometer-scale resolution—well beyond the ~250 nm diffraction limit of conventional light microscopy. At the Institute of Applied Optics and Biophysics (FSU Jena), SMLM is a core method for investigating the nanoscale organization of cells, membranes, and intracellular transport systems.
How it works: Circumventing the diffraction limit with time and statistics
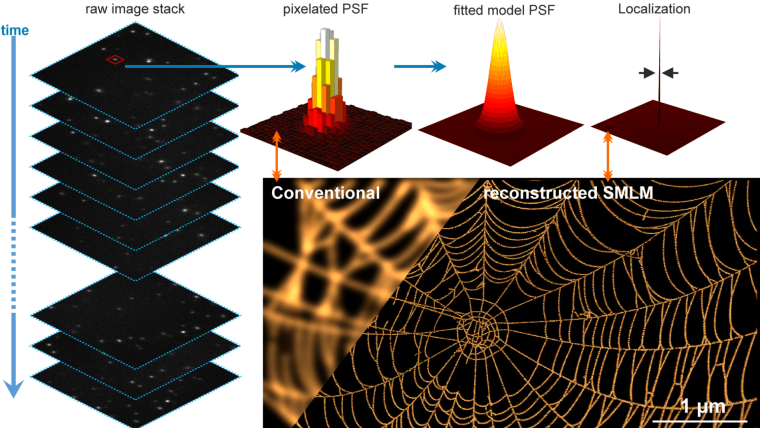
The key idea behind SMLM is deceptively simple: instead of trying to resolve many densely packed fluorophores at once (which would appear as a blurry, unresolved spot), SMLM activates only a sparse, random subset of fluorescent molecules at any given time. Each of these isolated emitters is then imaged, and its precise position is determined by fitting the point spread function (PSF)—typically to a 2D Gaussian. After repeating this process thousands of times and compiling all localized positions, a super-resolved image is reconstructed.
There are several widely used SMLM techniques, including:
- PALM (Photoactivated Localization Microscopy): uses photoactivatable fluorescent proteins.
- dSTORM (direct Stochastic Optical Reconstruction Microscopy): relies on dye pairs that can switch between dark and bright states.
- DNA-PAINT: exploits transient binding of fluorescently labeled oligonucleotides to achieve precise localization.
Regardless of the labeling strategy, the resolution achievable with SMLM is typically 10–30 nm laterally, and with suitable 3D extensions (e.g., astigmatism or biplane imaging), axial resolution can often reach < 50 nm.
Applications in biophysics and medical photonics
At IAOB, we apply SMLM to understand processes such as intracellular nanoparticle trafficking, cytoskeletal dynamics, and protein clustering in membranes, amongst many others. In CRC PolyTarget, for example, we use multi-color 3D SMLM to track how nanoparticles interact with organelles at the single-molecule level.
Why it matters for physicists
SMLM is a perfect example of how concepts from statistical optics, nonlinear photophysics, and computational modeling converge to push the boundaries of what’s observable. From a physics perspective, SMLM raises fascinating questions about localization precision, noise modeling, optical design, and inverse problems.
If you're interested in topics like:
- Point spread function engineering
- Bayesian inference and machine learning in microscopy
- Time-resolved molecular dynamics in living cells
- Instrument design for nanoscopy
... then SMLM (and other super-resolution microscopy approaches) offers a rich, interdisciplinary field to explore—from the lab bench to advanced data analysis.
Structured Illumination Microscopy
Coming Soon...