Jun.Prof. Dr. Christian Franke
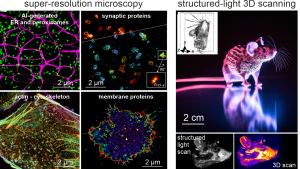
quantitative Single-Molecule Localization Microscopy is the core of our work.
Attention
News
X (the webside formerly known as Twitter)External link
- 10.11.2025: Linda from Gymnasium Hankensbuettel is visiting us during her internship in Jena. Welcome!
- 18.09.2025: Our NanTex manuscript on computational multiplexing and phenotyping of super-resolution microscopy data is now online at biorxivExternal link Congratulations to Bela and Gregor!
- 15.09.2025: Zineb and Eduard are joining the group! They will both strengthen our 3D team. Zineb will work on her Bachelors thesis and Eduard joins as a programmer. Welcome!
- 01.09.2025: We recieve funding from the FSU K1-Startup Service to build a showcase Gentschinator3000. What's the Gentschinator3000 you ask? News and releases will follow soon.
- 19.05.2025: Andreas' paper on Applying telecentric stereo 3D-measurement to small Lepidopterans - bridging the macro and the microscale with isotropic micrometer resolution is now online as preprint at biorxivExternal link Towards subcellular resolution in centimeter FOVs in whole animals.
- 24.04.2025: Our collaboration with the Schubert Lab on novel Lipid Nanoparticle Formulations is now published in SmallExternal link We could contribute triple-color super-resolution of LNPs in cellular context with nanometer resolution. Very cool collaboration - congratulations to all authors, especially Caroline who pushed it through!
- 22.01.2025: Gregor wins (again!!!) the 2025 PicoQuant Best Paper Award at the Photonics West in San Francisco! Congratulations!
- 21.01.2025: Christoph's paper on "Photometrically optimized event-based stereo 3D measurements" is now published in Optics ExpressExternal link Congratulations!
- 01.01.2025: Our collaborative Research-Group AI-supER has officially started its work, funded by the Thüringer Aufbaubank (TABExternal link) and the EU (ESF+)(total of 1 Mio. Euro over 3 years). Together with the groups of Christian Hübner (Hübner LabExternal link) and Michael Habeck (AG HabeckExternal link) from the University Hospital Jena we will develop advanced super-resolution microscopy and AI-based analysis tools to investigate the change of nanostructure of the enoplasmatic reticulum in neuro-degenerative deseases. Press Release via FacebookExternal link
-
News Archiv
- 10.07.2024: News release by the FSU regarding our BMBF-funded consortium 3DVens
- 01.05.2024: We got new funding! After almost 2 years of preparation, our BMBF-funded (total of 3 Mio. Euro over 3 years) consortium 3DVens starts in collaboration with Fraunhofer IOF and 3 industry partners. 3DVens will develop highly integrated Hardware and Software for a precise 3D colour Endoscope that can safely operate in explosive environments. Our Dr. Andreas Stark will function both as project leader and the consortiums scientific coordinator.
- 10.04.2024: We contributed to a great article in Nature Communications on the link between the visual and tactile circuit in mice. Read the Full articleExternal link Weiler, S., Rahmati, V., Isstas, M. et al. A primary sensory cortical interareal feedforward inhibitory circuit for tacto-visual integration. Nat Commun 15, 3081 (2024)
- 2023/03/17: Andreas was awarded 10.000 Euro by the IMPULSEproject of the FSU to push his research on multiscale structured illumination to the next level. Congratulations!
- 2023/03/05: Nabil successfully defended his outstanding Master Thesis with OQmentedExternal link and will continue his journey to become an outstanding optical designer. Congratulations and good luck in the future!
- 2023/02/01: We got funding from the LIFE 'Talent' program for hiring a research assistant - come join our team!
- 2022/12/01: Our work on Lipid Nanopraticles for mRNA delivery started in Dresden @MPICBG is featured in The Year in Cell Biology: 2022External link of the Journal of Cell Biology. We are building on this work with our project in the CRC Polytarget analysing the nanoscale cellular distributing of polymeric nanoparticles and their cargo. Our fantastic student Lukas Harder is pushing this together with polytarget partners.
- 2022/11/04: Our manuscript with the Holthoff Lab @UKJExternal link is now online at @biorxivExternal link. Andreas managed to tune his #structuredillumination based #stereophotogrammetry system to record mice whiskers with 3D micrometer resolution for the first time. This wat, we could contribute to understand the ovelap between the visual and tactile field of view in mice. Fantastic paper and much more to come from Andreas in this direction. Congratulations!
- 2022/10/15: Kristina and Matthew are joining us for their Research Labworks and Master Thesis'. Kristina will work with Andreas and the Holthoff Lab @UKJExternal link to further optimize our new structured illumination based 3D reconstruction approach of mice whisker. Matthew is working on rel time computation of large 3D point cloud data sets, both for microscopy and 3D measurements. Welcome and have Fun!
- 2022/08/25: Gregor has been selected for this years EMBO Practical Course Computational Optical BiologyExternal link in Oeiras, Portugal and awarded a Travel Grant from EMBO! Congratulations and have fun!
- 2022/08/17: Steev successfully defended his Master Thesis on smart glasses with Tooz Technologies and will continue working with them as a full time employee. Congratulations and all the best!
- 2022/07/18: Andreas' paper on "Miniaturization of a coherent monocular structured illumination system for future combination with digital holography" is now published in Light: Advanced Manufacturing. Congratulations! Much more to come soon. paperExternal link
- 2022/07/01: Aninidita from @AgEggelingExternal link and our very own Dr. Andreas Stark where awarded for their outstanding PhD works. Anindita got the Zeiss 'Ph.D. Award in Modern Optics' and Andreas the 'Ph.D. Award of Dr.-Ing. Siegfried Werth Foundation'. Congratulations! #IAOBpowerExternal link
- Anindita works at #IAOBExternal link and @Leibniz_IPHTExternal link as a PhD student on the development of multi-modal #SuperresolutionMicroscopyExternal link methods and Andreas is a postdoc with us and works on multi-scale optical measurements by structured illumination. Many great things to come from these two!
- 2022/06/01: Nabil joins the group and will work on augmented reality glasses for his Master Thesis with OQmented. Welcome!
- 2022/04/15: Liju joins us as for his Research Lab work on Pattern Optimization for Structured Illumination. Welcome!
- 2022/04/15: Kedar joins us as for his Research Lab work on 360 degree reconstruction. Welcome!
- 2022/04/07: Friedrich joins us as for his Bachelor Thesis on super-fast Pattern Creation for Structured Illumination. Welcome!
- 2022/04/01: Andrey joins us as a Research Assistant and will work on Computer Vision and Machine Learning for Super-Resolution Microscopy. Welcome!
- 2022/04/01: Lukas joins us as a Research Assistant and for his Bachelor Thesis on super-high resolution imaging of endosomal compartments. Welcome!
- 2022/03/18: Our recent paper on mRNA escape from endosomal recycling tubules, analysed by multi-colour dSTORM in the Journal of Cell Biology is featured in this weeks issue of Science! Wow! #mindblown
- 2022/03/15: Andreas' paper on "Miniaturization of a coherent monocular structured illumination system for future combination with digital holography" has been accepted in Light: Advanced Manufacturing. Congratulations! Much more to come soon.
- 2022/03/09: Our straight-forward & accessible-for-all workflow to quantitatively map and analyse the 3D-distribution of (T-) cell surface receptors with #isotropic nanometer resolution, while preserving the native membrane topography is now published in Communications BiologyExternal link
- 2021/11/01: Andreas joins the group as a postdoctoral Researcher after successfully finishing his PhD with Prof. Kowarschik. Andreas is an expert in macroscopic 3D measurement based on structured illumination and will broaden the scope of the group. Fun things to come I am sure! Welcome!
- 2020/11/01: Gregor gives the new Franke Lab a kick start in joing me right away. Gregor is starting his PhD thesis #FirstPhDStudent. Welcome and have fun - let's do this!
- 2020/11/01: First Day in the Office @FSU - #Exciting #NewPI
Single-Molecule Blinking of Fluorescent Dyes
Video: Christian FrankeSingle-Molecule Blinking of Fluorescent Dyes - Every spot represents a nanometer size dye molecule, imaged in a wide-field microscope onto a EMCCD chip. Detecting single molecules allows us to create super-resolution fluorescence images way beyond the Abbe diffraction limit.
Institute of Applied Optics and Biophysics - Group Experimental Digitized Microscopy
Helmholtzweg 4
07743 Jena
Google Maps site planExternal link
Postal address:
Friedrich-Schiller-Universität Jena
Institut für Angewandte Optik und Biophysik
Max-Wien-Platz 1
07743 Jena